Crohn’s disease affects over 150,000 Canadians, and up to half will develop fistulas. Fistulas are painful, abnormal tunnels between the intestine and other organs that are notoriously difficult to treat. My research uses human intestinal organoid technology to understand how these fistulas form, with the goal of identifying new therapeutic targets to improve patient outcomes.
The Problem: Why Fistulas Matter
Fistulas are one of the most debilitating complications of Crohn’s disease. These abnormal tunnels penetrate through the gastrointestinal tract to connect with other organs and surfaces, causing ongoing pain, repeated infections, and significantly reduced quality of life.
Despite their clinical significance, the biological mechanisms driving fistula formation remain poorly understood. A critical barrier to progress is the absence of physiologically relevant models that can accurately replicate the human intestinal environment and the complex cellular changes that occur during fistula development.
Research Approach
My research uses human intestinal organoids—three-dimensional “mini-guts” grown from patient intestinal tissue biopsies. These organoids replicate the structure, cellular diversity, and function of the human intestine, providing a platform to study disease mechanisms in a physiologically relevant context.
My research is conducted under the supervision of Dr. Simon Hirota, Professor at the Cumming School of Medicine, and co-director of the Human Organoid Innovation Hub (HOIH) and Canadian National Organoid Network (CNON). Through these networks, I have access to patient-derived samples from both Crohn’s disease patients and healthy donors. This allows me to directly compare disease and healthy tissue responses and account for biological variables including sex-based differences in organoid behavior.
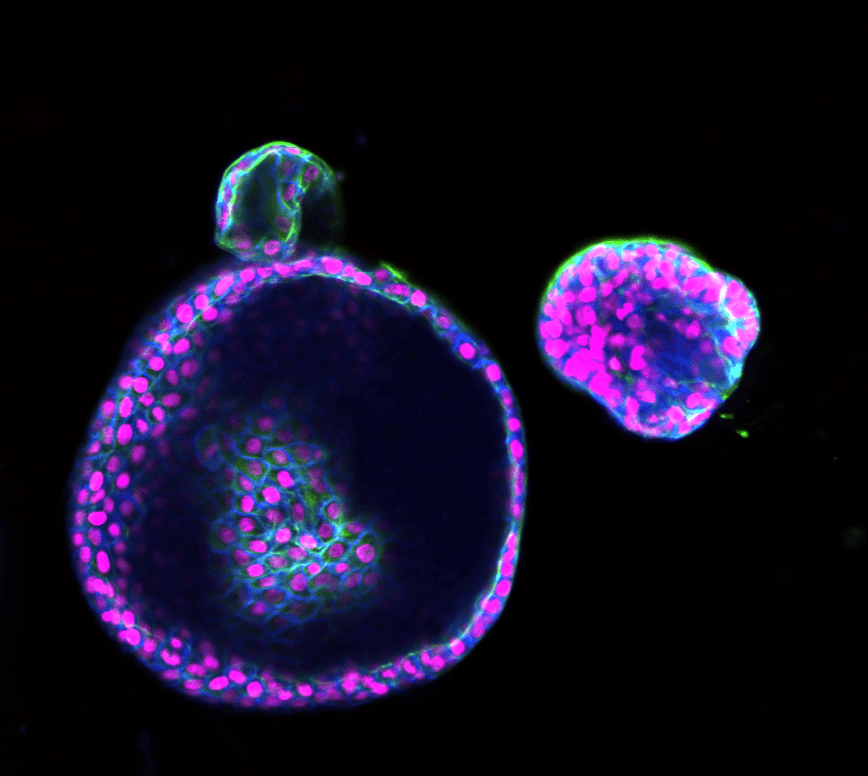
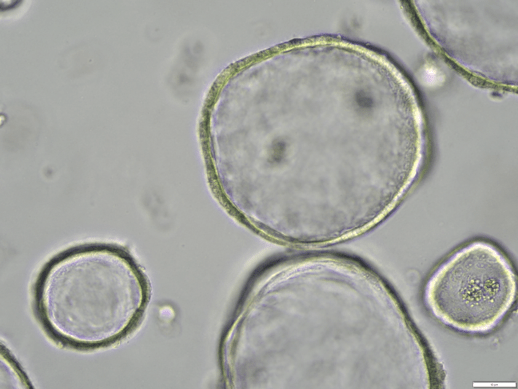
Studying Epithelial-to-Mesenchymal Transition (EMT)
In a healthy intestine, epithelial cells maintain strong connections through proteins like E-cadherin, forming the intestinal barrier. In contrast, Crohn’s disease fistulas are lined with transitional cells that have undergone epithelial-to-mesenchymal transition (EMT). EMT is a process where epithelial cells lose their structural identity and gain the ability to migrate and invade surrounding tissues.
My project involves exposing intestinal organoids to inflammatory molecules enriched within Crohn’s disease fistulas and monitoring how the organoid’s shape, internal structure, and gene expression change. To track EMT in real-time, I am developing an innovative fluorescent reporter system using adeno-associated virus (AAV) technology. When EMT-related genes activate, the organoids will express fluorescent signals that I can quantify using live-cell imaging.
Broader Impact
This research addresses a significant unmet clinical need in Crohn’s disease patient care. The organoid-based model I’m establishing will be adaptable for studying a range of gastrointestinal diseases affecting the intestinal epithelium.
Beyond understanding disease mechanisms, this platform has potential applications in therapeutic screening and personalized medicine approaches. By working with patient-derived samples, we can begin to understand disease heterogeneity and identify why different patients respond differently to treatments.
This work is supported by funding from CIHR and Crohn’s and Colitis Canada.
